Matt Peerlings
PhD candidate
Supervisor:
Promotor:
Employed since:
Sponsor:
dr. Peter Ngene
prof. dr. Petra de Jongh
December 2020
NWO, since 2020
Email:
Room:
4th floor study area DDW
Studying the Stability of Electrocatalysts for CO2 Reduction
Rising levels of CO2 in the atmosphere cause climate change and must therefore be mitigated. One option to convert CO2 is the electrochemical CO2 reduction reaction into valuable chemicals. For this, suitable electrocatalysts are required that possess high activity, selectivity and stability. Different metals have been investigated, depending on the product of interest. Typical products include CO (on Zn, Ag and Au) and HCOOH (on Sn, Bi and Pb). Some metals are inactive for CO2 reduction and hence only produce H2 in the main competing hydrogen evolution reaction. Especially interesting is Cu, because it is the only metal that can produce valuable hydrocarbon and alcohol products like ethylene and ethanol in significant amounts. However, significant improvements in the selectivity to these so-called C2+ products are required before industrial applications can be feasible.[1]
Previous research has shown that the selectivity of Cu-based catalysts can be improved by use of oxide-derived copper, nanostructuring and tuning the catalyst shape.[2] Additionally, binders can be added to tune the catalyst micro-environment.[3] An especially interesting approach for enhancing the C2+ product selectivity is addition of a CO producing metal like Zn, which can increase the CO coverage on the copper surface and thereby improve the formation of C2+ products.[4] However, studying the relative contributions of these different catalyst properties is by no means trivial, as the electrocatalysts often change significantly under reaction conditions.
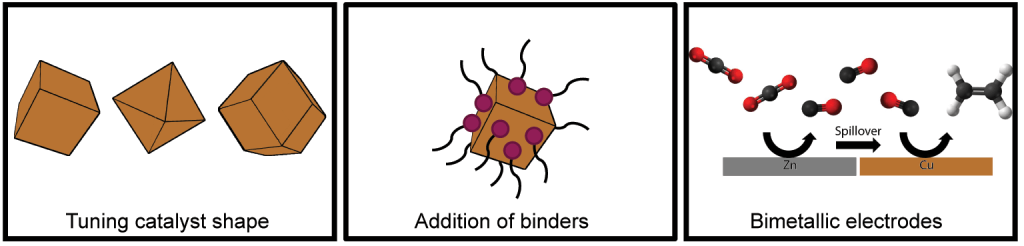
In this thesis, which is part of the RELEASE consortium, different strategies like the aforementioned ones are investigated for improving the performance of Cu-based electrocatalysts for the CO2 reduction reaction. The focus lies hereby on understanding the changes occurring to the catalysts under reaction conditions, especially at long testing times. By doing this, we aim to gain more knowledge on the relative contributions of the different strategies to ultimately design more selective and stable Cu-based electrocatalysts for CO2 reduction.
References
[1] Y. Hori et al., Modern Aspects of Electrochemistry 42, 89-189 (2008)
[2] S. Nitopi et al., Chemical Reviews 119, 7610-7672 (2019)
[3] J. Bui et al., Accounts of Chemical Research 55, 484-494 (2022)
[4] A. da Silva et al., ChemCatChem 880, 114750 (2021)
CV
2020 – present
PhD candidate in the group of prof. dr. Petra de Jongh and dr. Peter Ngene at Materials Chemistry and Catalysis
2018 – 2020
Master’s degree in Nanomaterials Science at Utrecht University.
Master thesis at the Inorganic Chemistry and Catalysis group under supervision of prof. dr. Petra de Jongh, dr. Peter Ngene and Laura de Kort, titled “Effects of nanoconfinement on the ion conduction properties of Li-based electrolytes for all-solid-state batteries.”
Internship at BASF de Meern
2014 – 2018
Bachelor’s degree in Chemistry at Utrecht University.
Bachelor thesis at the Condensed Matter and Interfaces group under supervision of prof. dr. Andries Meijerink, titled: “Temperature Dependence of Quantum Dot Emission Linewidths”