Harsh Wadhwani
PhD candidate
Supervisor:
Co-promotor 1:
Co-promotor 2:
Employed:
prof. dr. Petra de Jongh
dr. Peter Ngene
dr. Ben Erné
July 2025 – present
Email:
Room:
Sponsor:
h.m.wadhwani@uu.nl
DDW 4th floor Open area
ANION Consortium
3D porous model systems for electrocatalysis
The ever-rising CO2 emissions have been detrimental for the climate on the earth causing a surge in average global temperatures. The global temperatures have already increased by 1oC with current atmospheric CO2 concentrations of 422.8ppm [1]. To prevent the temperatures from rising further, it is important to control the release of CO2 into the atmosphere and to utilize the CO2 already present using various CO2 utilization technologies. One of the ways to use this CO2 is to convert it electrochemically to useful chemicals like CO, CH4, C2H4, CH3OH, C2H5OH, etc. This has been a topic of research since 1999 with the first evidence by Hori et.al.[2] However, performance of this process is not yet at the industrial scale, with one of the reasons being the lower current densities and faradaic efficiencies for specific products like C2H4.
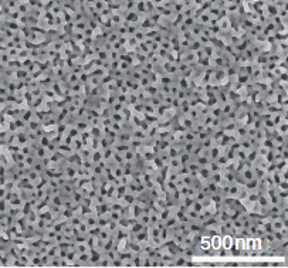
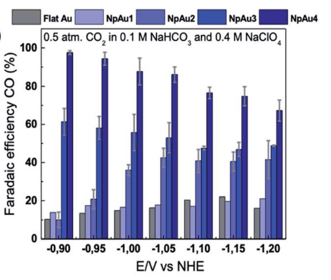
One of the ways to increase the current density and selectivity is to use catalysts with very high electrochemically available surface areas. Increasing the size of the electrode is not a viable option since it can only be done until a certain limit due to system level considerations. However, making the electrode porous instead of flat can increase the surface area drastically, with the same geometric surface area [3]. The surface area relates inversely to the pore size. However, with decreasing pore size, the mass transport of different species like ions, solvent molecules and dissolved gases become increasingly difficult leading to lower activity.[4,5] Such structures are also shown to enhance selectivity for one specific product due to mass transport limitation. [6] More complexities arise due to charging of the pores under operation making it difficult for ions of same charge to enter the pores.
The goal is to create mesoporous films (2–50 nm pores) using soft templating for uniform pore sizes, and combined templating to produce hierarchical pore structures on conductive substrates such as metal foils or carbon paper. Such porous electrodes will be used to study how nanoconfinement affects physical properties of electrolyte species such as solubility, due to the electrostatic interactions of ions and solvent molecules with the charged solid surfaces. They will also help investigate ion mobility and mass transport (e.g., solvent and CO2 diffusion) under applied potentials.
We will use also advanced microscopy techniques like cryoTEM and in-situ TEM to visualize metal cations in the pores. We will also assess mass transport by comparing the porous structure’s electrical resistance with resistance from ohmic heating under faradaic operation, using electrical impedance spectroscopy and microcalorimetry.
This project is a part of the ANION consortium which focuses the mission to bring together the advanced tools from the various chemistry and physics disciplines, such that we may arrive at a deep microscopic understanding of electrochemical interfaces, enabling an holistic rational design of new electrochemical devices, encompassing all relevant aspects of nanoscale electrochemical charge transfer.
References
[1] Change, N. G. C. (n.d.). Carbon dioxide concentration | NASA Global Climate Change. Climate Change: Vital Signs of the Planet. https://climate.nasa.gov/vital-signs/carbon-dioxide/?intent=121
[2] Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media, Electrochim. Acta, 1994, 39, 1833–1839.
[3] S. Suter and S. Haussener, Optimizing mesostructured silver catalysts for selective carbon dioxide conversion into fuels, Energy Environ. Sci., 2019, 12(5), 1668–1678.
[4] Ittersum, M. E. T. V., Betz-Güttner, E., Hellebrand, E., Keijzer, C. J., Peerlings, M. L. J., Ngene, P., & De Jongh, P. E. (2025). Quantification of the porosity in template-based ordered porous Ag electrodes and its effect on electrochemical CO2 reduction. Reaction Chemistry & Engineering.
[5] A. Goyal, C. J. Bondue, M. Graf and M. T. M. Koper, Effect of pore diameter and length on electrochemical CO2 reduction reaction at nanoporous gold catalysts†, Chem. Sci., 2022, 13(11), 3288–3298.
[6] K. D. Yang, W. R. Ko, J. H. Lee, S. J. Kim, H. Lee, M. H. Lee, K. T. Nam, Morphology-directed selective production of ethylene or ethane from CO2 on a Cu mesopore electrode. Angew. Chem. Int. Ed. 56, 796–800 (2017).
C.V.
July 2025-Present
PhD candidate in the Materials Chemistry and Catalysis group at Utrecht University under the supervision of prof. dr. Petra de Jongh.
2022 – 2024
MSc Chemical Engineering (Hons.), TU Delft
Thesis: Bicarbonate and Nitrate co-electroreduction to urea in a water-fed PEM electrolyzer, in the group of Prof. Atsushi Urakawa.
Internship: Dow Benelux, Terneuzen. Stability of Pt single atom catalysts under high temperature dehydrogenation reactions.
2018 – 2022
B.Tech Oils Oleochemicals and Surfactants Technology.
Thesis: Synthesis of guerbet alcohols from oleic acid using Ru based catalysts.